Enamel matrix derivative in conjunction with bone grafts
|

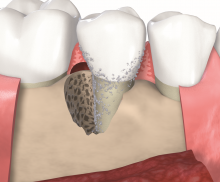
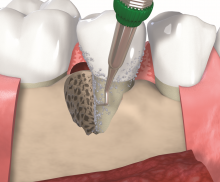
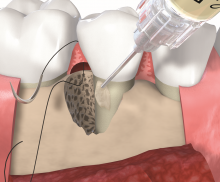
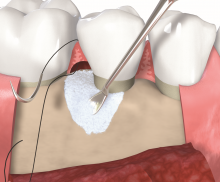
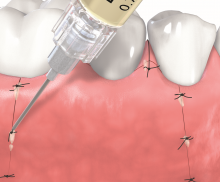
Non-contained intrabony defects (2- or 1-wall defects) usually require volume-providing grafting material in order to prevent flap collapse and to support the soft tissue. Following flap elevation and granulation tissue removal, the root surfaces are freed from plaque and calculus. The remaining smear layer is removed by applying Straumann® PrefGel® for 2 minutes. After thorough rinsing, Straumann® Emdogain® is applied onto the root surfaceto to fully cover the exposed root. The osseous defect is filled with bone substitute particles that were pre-mixed with Straumann® Emdogain®. To support soft tissue wound healing, a final layer of the gel is applied on top of the bone graft before final wound closure.
Different kind of bone substitute materials may be used to fill the defect. cerabone® is a natural bovine bone graft consisting of pure bone mineral. Due to its natural bone structure, cerabone® provides optimal support for bone forming cells and blood vessels. A further alternative are the the allogenic maxgraft® granules or the synthetic material maxresorb®. maxresorb® is a biphasic material composed of 60% hydroxyapatite and 40% beta-tricalcium phosphate. While the fast resorption of beta-TCP quickly offers space for new bone formation, the HA component provides volume stability for an extended time period.