Bone ring technique
|

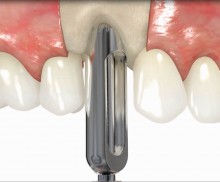
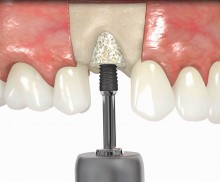
botiss offers prefabricated allogenic bone rings, whose use minimizes the risks associated with the harvesting procedure and reduces the post-operative pain as well as the risk of infection and operation time. maxgraft® bonering is a sterile, highly safe allograft product derived from the femoral heads of living human donors. The prefabricated rings can be placed press-fit into the prepared ring bed.
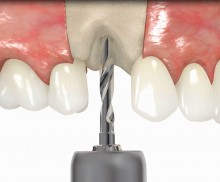
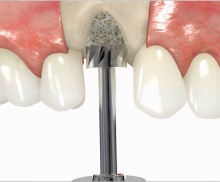
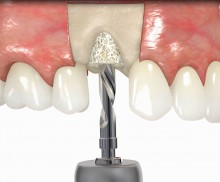
In addition, botiss offers a surgical kit that provides all necessary instruments for the bone ring technique. The first step of the surgical procedure is the determination of the position of the implant using the pilot drill; the ring bed is then prepared with a trephine. Subsequently, the use of a planator enables an even paving of the local bone to achieve an optimal contact with maxgraft® bonering as well as the removal of the cortical layer, which improves the graft revascularization. With maxgraft® bonering, a vertical bone augmentation of 2–3 mm above bone level can be easily performed. For the augmentation of a severely atrophic mandibular, a width of the ridge (in the case of a parallel-walled ridge) of at least 4 mm is necessary for a successful application of maxgraft® bonering. The technique is also applicable for a one-stage sinus lift with minimal maxillary bone height. In this case, the implant is fixed in the sinus with the allogenic bone ring; these are held together with a membrane screw.
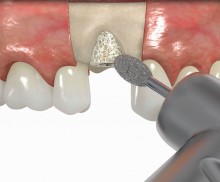
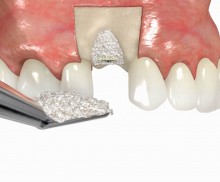
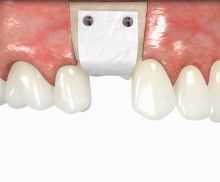
It is recommended to fill the residual defect volume with a slowly resorbable bone regeneration material (i.e., cerabone®) and to cover the augmentation site with a collagen membrane. The pericardial Jason® membrane is an ideal solution in such a situation due to its long-term barrier function. A tension-free suturing of the operation site minimizes the risk of tissue perforation and/or graft exposure.
Please Contact us for Literature.