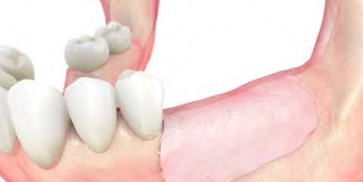
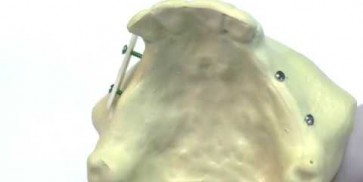
maxgraft® cortico
- Vertical augmentation
- Horizontal augmentation
- Complexe three-dimensional augmentations
- Single tooth gaps
- Fenestration defects
|
- Established augmentation technique with new material
- Significant reduction of operation time
- Standardized, unform thin struts
- No donor-site morbidity
- No limitation in availability for larger augmentative interventions
Art.-No. | Dimensions | Content | ||
|---|---|---|---|---|
BO-31251 | 25x10x1 mm | 1x cortical strut | ||
BO-34000 | cortico trimmer | |||

The proper size of the plate is estimated after the elevation of the mucosal flap or preoperatively using a digital planning software. Using a diamond disc, the plate is then cut extraorally. The plate is positioned within a certain distance by predrilling through the plate and local bone; fixation is performed with osteosynthesis screws to create a fixed compartment. To prevent the perforation of the soft tissue, the sharp edges have to be removed, e.g. by using a diamond ball. The space between local bone and cortical plate can be filled with a variety of different particulated bone grafting materials. Then, the augmentation area needs to be covered with a barrier membrane (Jason® membrane, collprotect® membrane) and a tension-free and saliva-proof closure must be applied.
Please find our free webinars at www.botiss-webinars.com
Kostenfreie Webinare zu Schulungszwecken finden Sie unter www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please find our free webinars at www.botiss-webinars.com
Please Contact us for Literature.